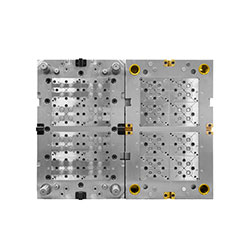
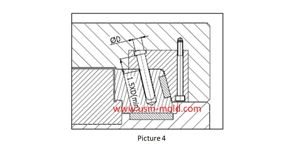
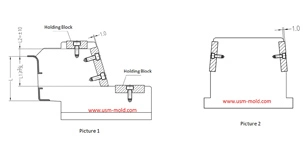
Medical Pipette Tip Mold
USM as a medical plastic injection mold supplier provides products including medical device plastic parts, suction tips, blood collection devices, pregnancy test sticks, culture tubes, ventilator masks, etc. All plastic parts which will be used in the medical industry are all called medical plastic products.
Medical Pipetting Tips Mold Characteristics
Due to the contact with liquid medicine or the human body, the basic requirements of medical plastics are chemical stability and biological safety. To put it simply, the components in the plastic material can not be precipitated into the liquid medicine or the human body, will not cause toxicity and damage to the tissues and organs and are non-toxic and harmless to the human body. Medical plastic injection molding products usually sold on the market are certified and tested by medical authorities In order to ensure the biological safety of medical plastics, and users are clearly informed which grades are medical-grade, medical plastics material in the United States usually pass FDA certification and UsPVI biological testing.
 English
English русский
русский